Isotope Analysis: Dating and Diet
Dating and Diet of the Human Skeletal Remains from the Iyai Rock Shelter Site
In addition to observable morphological information, ancient human skeletal remains retain a variety of information in their chemical composition. Approximately a quarter of living bone comprises organic matter, and under suitable conditions for chemical analyses, a few percent of this may be preserved in archaeological human skeletal remains. While hydroxyapatite, the inorganic bone component, undergoes replacement during the fossilization process, organic matter retains components produced during the individual’s life, providing a wealth of information. Here, we present analysis conducted on collagen, the primary organic component of bone. Specifically, by measuring isotopes of major components such as carbon and nitrogen, it’s possible to determine the age of skeletal remains and the types of food consumed by individuals.
Isotopes are atoms sharing the same chemical properties but differing physically in mass. Carbon has three known isotopes with masses of 12, 13, and 14, while nitrogen has two known isotopes with masses of 14 and 15. Naturally occurring carbon consists mainly of carbon 12 (12C) (99%) and carbon 13 (13C) (1%), while nitrogen consists mainly of nitrogen-14 (14N) (99.6%) and nitrogen-15 (15N) (0.4%). Despite identical chemical properties, proportions of isotopes (isotope ratios) vary among substances due to different reaction rates of different masses. Differences in carbon and nitrogen isotope ratios exist in animals and plants presumed to have been consumed by Jomon period people from the Iyai rock shelter site (Fig. 1). As carbon and nitrogen isotope ratios in animal tissues strongly reflect the ratios in food proteins, it’s possible to ascertain the diet of an individual by analysing carbon and nitrogen isotope ratios in collagen from their skeletal remains.
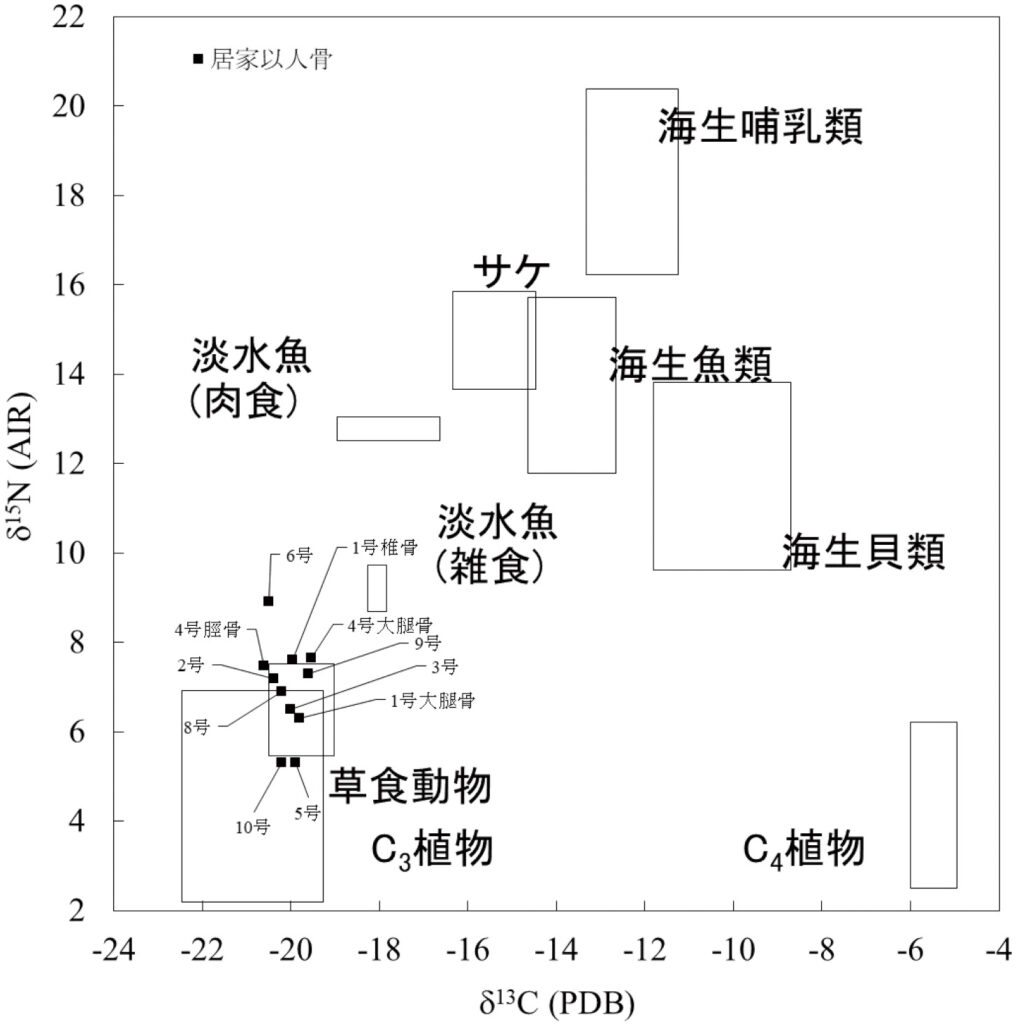
from the Iyai site and major food resources
For instance, terrestrial animals and plants exhibit low concentrations of heavier carbon (carbon-13) and nitrogen (nitrogen-15). Most human skeletal remains from the Iyai rock shelter site share similar properties to those of terrestrial animals. Wild cereal species (C4 plants) with high carbon-13 content were found in the site’s ash deposit, yet these properties are not observed in the human skeletal remains. It’s inferred that marine products, high advances in carbon-13 and nitrogen-15, were rarely part of the diet of individuals at the Iyai rock shelter site. If marine products were consumed, even seasonally, evidence should be present in bone isotope ratios, considering it takes over a decade for bone components to replace. No such trend indicating consumption of salmon migrating upriver from the sea is evident. Hence, it’s assumed that the Iyai populations primarily consumed local terrestrial animals and plants.
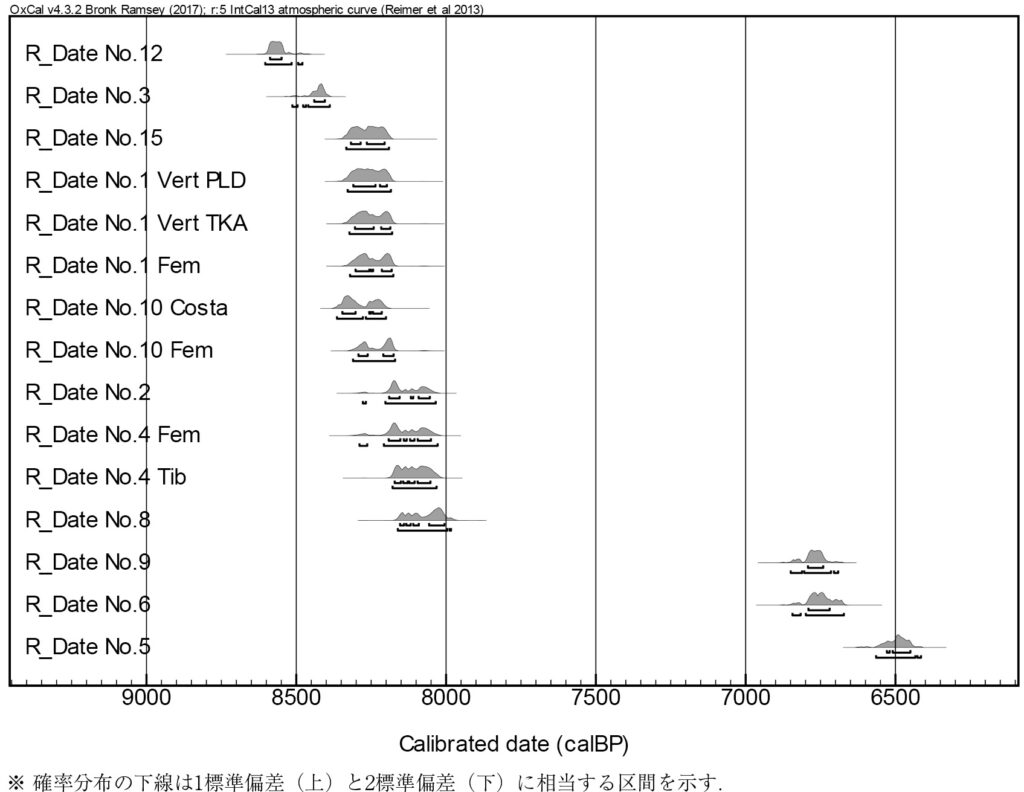
Moreover, radiocarbon (14C) present in collagen can be measured to determine the period when the individual lived. 14C, which has an unstable nucleus, decays over time through radioactive decay, and is turned to nitrogen. Although 14C exists in minuscule amounts during life – one per trillion carbon atoms – its proportion halves every 5730 years (known as the half-life). Accelerator mass spectrometry (AMS) is used to measure this minute quantity of 14C, accelerating ions at high voltages. With this equipment, dating as little as one milligram (one thousandth of a gram) of carbon is feasible. Bone fragments weighing less than one gram have also been dated, and to date, 11 individuals from the Iyai rock shelter site have been dated. The results indicate dates in both the Initial Jomon period (8500-8000 years ago) and the Early Jomon period (6500-6000 years ago), suggesting the site was utilized for burials in both periods (Fig. 2). Notably, upper and lower bodies discovered separately in some burials share matching dates, confirming they belong to the same individual. Additionally, no significant changes were observed in carbon and nitrogen isotope ratios, indicating dietary continuity between the periods despite over 1000 years having elapsed.
Place of Origin Investigated through Strontium and Oxygen Isotopes
Additional research involves analysing the isotope ratios of oxygen and strontium in tooth enamel. Permanent teeth are unique as they are formed during childhood and retain their original composition throughout life. Enamel’s robust crystalline structure makes it less susceptible to soil contamination during burial, enabling analysis of components in the inorganic hydroxyapatite, which is unfeasible in typical bone remains.
The properties of enamel can be used to obtain information on where an individual spent their childhood. Enamel crystals absorb strontium, which has similar properties to calcium. Strontium has several isotopes, including strontium-87 (87Sr) produced from radioactive decay of rubidium-87 in ancient rocks. Strontium isotope ratios (87Sr/86Sr) vary based on rock age and geology, and geological isotope ratios will be recorded in the teeth of the Jomon people. If an individual from a strontium-87-rich area migrates to a low-strontium-87 area, differences will persist in their teeth compared to those who grew up in the latter area.
Similarly, the proportion of oxygen-18 (18O) in oxygen varies by region. Oxygen-18 in rainwater changes with latitude, distance from the sea, altitude, etc. In conjunction with Sr isotopes, the proportion of oxygen-18 in the tooth enamel of an individual contains information on the region that they grew up in, which in turn should allow for estimates of their place of origin and reveal any migrations.
During this research, plants are being collected to study strontium-87 distribution in the vicinity of the Iyai rock shelter site. Although a new mapping method reflecting geological findings is being developed, we hope to integrate this with the archaeological findings in the near future and provide new information on human migration during the Initial Jomon period.
Minoru Yoneda